Abstract
Background : Invasive fungal infections (IFI) are life threatening infections reported mainly in acute leukemia patients with prolonged deep neutropenia and patients post bone marrow or organ transplants.
IFI are an uncommon consequence of CLL and NHL, cranial (brain, sinuses) involvement is distinctly rare. Epidemiological data regarding fungal infections in lymphoproliferative disorders are scarce. The Italian SEIFEM group reported the frequency of IFI in a retrospective multicenter study of CLL patients as 4 per 1104 patients over 5 years and among patients with NHL 27 per 3457 over 5 years. In a report combining 8 European countries, only 10 cases of CNS aspergillosis were identified in non-neutropenic ICU patients, between the years 2006-2011.
Ibrutinib is a kinase inhibitor effective in the treatment of CLL and other NHLs has also been shown to have inhibitory activity against macrophages essential for defense against molds and Cryptococcus species.
In 2016 we described 3 non-neutropenic CLL patients who developed invasive cranial aspergillosis while on treatment with ibrutinib (NEJM, 2016). In all three patients this developed shortly after beginning therapy with ibrutinib. All were on steroids before starting therapy and the course was fulminant. In two of the three patients the infection was fatal.
Recently Lionakis et al (Cancer Cell, 2017) reported invasive aspergillosis in seven of 18 (39%) PCNSL patients treated with ibrutinib. Sites of involvement were cranial, pulmonary or both. None had prolonged neutropenia. The authors suggest that ibrutinib impairs fungal immune surveillance which may be exacerbated by the combined use of steroids and/or chemotherapy.
In order to better understand if these cases were isolated events or connected to therapy with ibrutinib, in this retrospective, investigator initiated international study we summarize the clinical and laboratory parameters of patients who developed invasive fungal infections while being treated with ibrutinib.
Methods : Patients with IFI were identified by treating physicians. Clinical and laboratory data were collected and managed using REDCap electronic data capture tools hosted at Shaare-Zedek Medical Center1(doi:10.1016/j.jbi.2008.08.010). The study received an IRB approval.
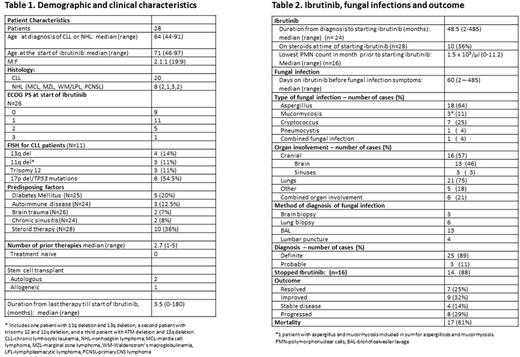
Results : Twenty-eight patients with IFI were enrolled from 15 centers in 6 countries by 18 physicians (Table 1). The median age at the start of ibrutinib treatment was 71, and 71% had CLL. The median number of prior therapies was 3. Six out of 11 (54%) of patients with FISH results had 17pdel/ TP53 mutations. Twenty patients (71%) had at least 1 predisposing factor, 36% were treated with steroids when starting ibrutinib and three patients were post bone marrow transplants. The median lowest neutrophil count in the month before starting ibrutinib was 1.5 x 103/µl. Aspergillus was the main pathogen (64% of the cases), the main site being the lungs (75%) and cranial (brain, sinuses) in 57%. Six patients had multiple sites of involvement. The diagnosis of the pathogen was definite in 25 (89%) and probable in 11%. IFI resolved in only 25% of patients and mortality was 61% from all causes.
Discussion : This cohort of 28 patients developed IFI on therapy with ibrutinib in the absence of common risk factors. Other unique features of our cohort are the high prevalence of CNS involvement (almost half of the patients); and the presentation of Aspergillus and Mucor ycosis spp infections at multiple sites, which is not a typical presentation for these pathogens. As the differential diagnosis was wide, most patients went through an invasive diagnostic procedure and therefore a high rate of a definite diagnosis was achieved rapidly in comparison to acute leukemia patients. Our preliminary results suggest that ibrutinib might be a risk factor for IFI resulting in unique patterns of clinical presentations. Subsequent comparative studies are urgently needed to better identify which patients on ibrutinib treatment are at greater risk for IFI.
Brown: Astellas Pharma: Consultancy; Sun BioPharma: Consultancy, Research Funding; Redx: Consultancy; Infinity Pharmaceuticals: Consultancy; Roche/Genentech: Consultancy; Janssen: Consultancy; Celgene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Honoraria; Pfizer: Consultancy; Janssen Oncology: Honoraria; Pharmacyclics: Consultancy; Gilead: Consultancy, Research Funding. Nijland: Novartis Pharmaeuticals Corporation: Honoraria; Kite Pharma: Honoraria; Roche: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; MIllennium/Takeda: Honoraria, Research Funding; Mundipharma: Honoraria; Gilead Sciences: Honoraria; BMS: Honoraria; MSD: Honoraria; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal